This week Mr. Cline’s 4th Block AP Chemistry class completed a two-day lab on titration.








Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction reaches neutralization, which is often indicated by a color change. It is an analytical lab technique where a known volume and molarity of a titrant is dispensed from a burette into a flask containing a measured quantity of the analyte. By using the blanched chemical equation specific to the reaction, moles of the titrant can be calculated at the equivalence point. The equivalence point is when the moles of the titrant and moles of the analyte are stoichiometrically equivalent. This means that the moles of titrant react with the moles of the analyte in a ratio determined by the blanched equation. In order to know when this point is reached, a color change is often used to signal the end point.
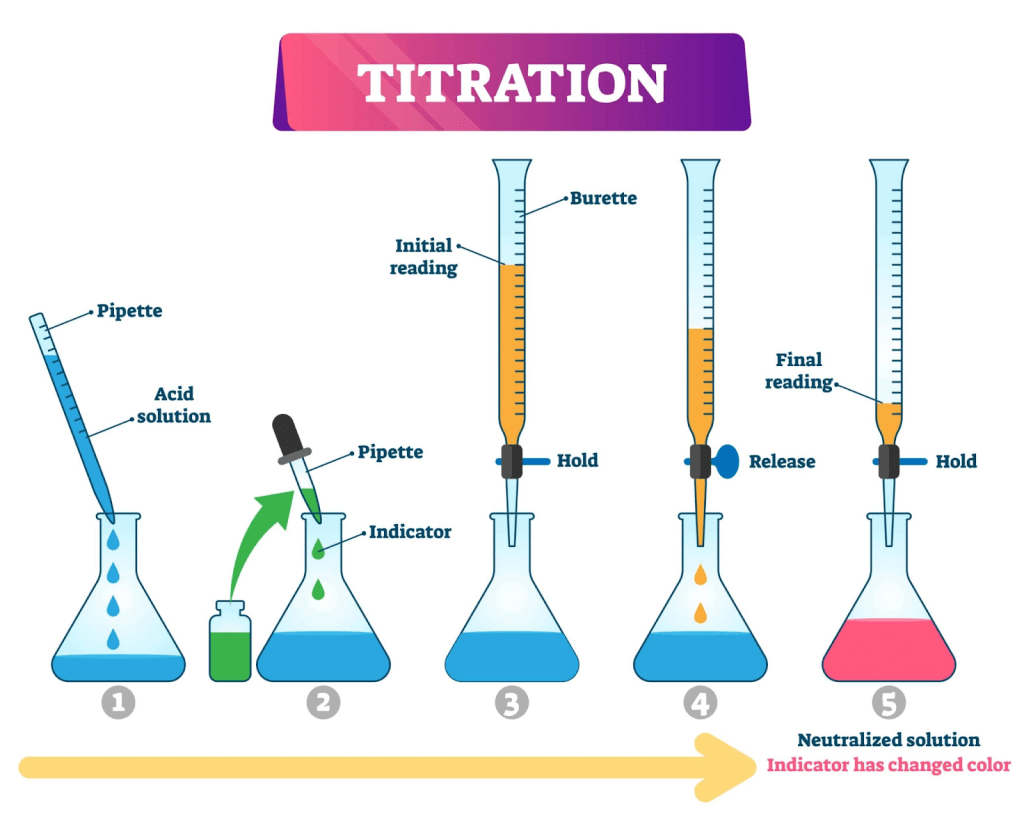
There are three common types of titrations:
- Acid-Base Titrations – either the acid or the base can be the titrant. An acid-base indicator which changes color at a pH close to 7 is added to determine the end point of a strong acid/base reaction. Sometimes pH meters are used to determine the equivalence point.
- Redox Titrations – during these reactions, a color change is produced when the oxidation state of a metal ion in the ration changes.
- Precipitation Titrations – when the titrant reacts with ions in the analyte, a precipitation can occur. The formation of a precipitate or its color change can signal the end point.
Equipment needed to perform a titration include:
- Burette for keeping track of titrant volumes
- Volumetric pipette for dispensing a specific volume of the analyte solution
OR
- Lab balance to mass a sample
- Erlenmeyer flask
- acid/base indicator OR
- pH meter




Leave a comment